Are You a Healthcare Professional?
This European website, initiated and developed by CSL Behring, contains product specific information and promotional content on gene therapy in haemophilia B and is intended for an international audience of European healthcare professionals only. For more detailed information on the use of the product in your country, please visit www.cslbehring.com.
Yes, I am a healthcare professional*
Enter SiteAbout HEMGENIX®
HEMGENIX® is a pioneering gene therapy that offers liberation from routine prophylaxis for a wide range of haemophilia B patients. It can provide long-term bleed protection and near-normal, long-lasting FIX levels with a one-time infusion.1,2

HEMGENIX® is the first and only approved gene therapy for haemophilia B in europe, addressing the root cause of haemophilia B and reducing its impact on patients.3
HEMGENIX® uses the non-replicating recombinant adeno-associated virus 5 (AAV5) vector for liver-directed introduction of a therapeutic FIX-Padua gene.1 This highly active FIX Padua protein variant is shown to generate 5-8 times higher mean endogenous FIX activity than the more common wild-type FIX protein.4,5,6
Liberation from routine prophylaxis1,2,*
96,3%
of patients discontinued routine FIX prophylaxis and remained prophylaxis-free1,*
-64%
annualized bleeding rate (ABR) reduction vs well-conducted prophylaxis in lead-in period1,**
36,9%
mean FIX activity at 1,5 years, and sustained at 2 years post infusion1,†
safety profile with no treatment-related serious adverse events1,‡
Gene Therapy For Haemophilia Explained
Haemophilia A and B are both monogenic, X-linked genetic disorders that are suitable for gene therapy.2,7 Gene therapy for haemophilia aims to increase clotting Factor VIII levels (haemophilia A) or Factor IX levels (haemophilia B), by providing a new functional gene or coding sequence, leading to improved health outcomes and reducing or eliminating the need for routine factor prophylaxis.2,8,9

Gene Therapy Mechanism Of Action Visual Infographic
See gene therapy for haemophilia broken down into 7 steps in this visual mechanism of action infographic, from AAV vector attachment to clotting factor protein synthesis.
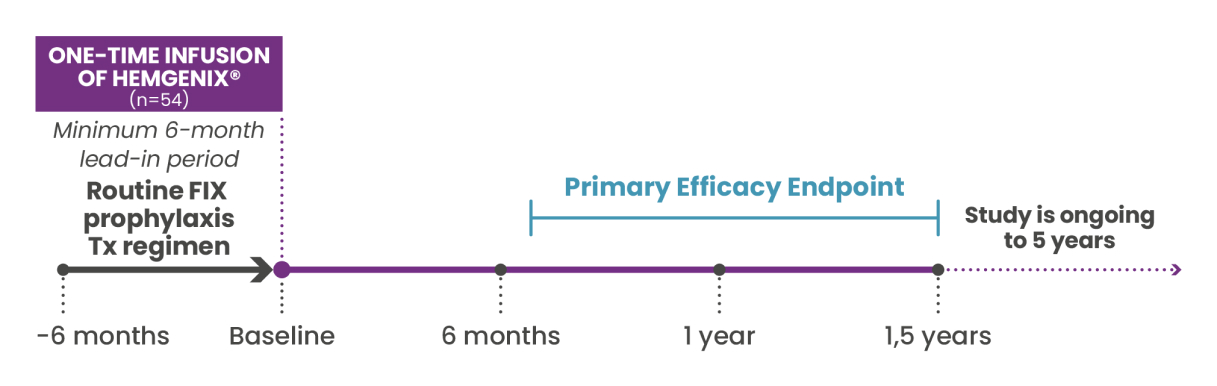
HOPE-B: A pioneering Phase 3 trial
The robust efficacy, safety and tolerability profile of HEMGENIX® was demonstrated in the multinational, pivotal Phase 3 HOPE-B trial.1,2
HOPE-B included 54 male patients ≥18 years of age with congenital haemophilia B (FIX activity ≤2% of normal), currently on continuous FIX prophylaxis for ≥2 months prior to screening.1,2
Primary Endpoint:1,2
Comparing annualized bleeding rate (ABR) for all bleeds between HEMGENIX® and prophylaxis for non-inferiority between the 6-month lead-in period and the 52 weeks following stable FIX expression (month 7-18).
Key secondary endpoints:1,2
- FIX activity at 6, 12, and 18 months after dosing
- Proportion of patients remaining free of continuous prophylaxis
- Occurrence and resolution of target joints, and proportion of patients with zero bleeds
- Correlation of FIX activity levels to pre-existing AAV5 NAb titre
- Adverse events
The primary endpoint has been completed, with patient data evaluated for the first 24 months following HEMGENIX® treatment. Patient follow up will continue for 5 years after administration of HEMGENIX®.1,2
Previous
Home
Next
Clinical Evidence
References
- EU SmPC HEMGENIX® (European Medicine Agency, 2023)
- Pipe SW, et al. Gene Therapy with Etranacogene Dezaparvovec for Hemophilia B. N Engl J Med. 2023;388:706-718.
- CSL Behring. First Gene Therapy for Hemophilia B, CSL's HEMGENIX®, Approved by the European Commission. Press release. 20 Feb 2023. Available at: https://newsroom.csl.com/2023-02-20-First-Gene-Therapy-for-Hemophilia-B,-CSLs-HEMGENIX-R-,-Approved-by-the-European-Commission. Accessed March 2023.
- Nathwani AC. Gene therapy for Haemophilia. Hematology Am Soc Hematol Educ Program. 2019;2019(1):1-8.
- Thornburg CD. Etranacogene dezaparvovec for hemophilia B gene therapy. Ther Adv Rare Dis. 2021;2:1–14.
- Von Drygalski A, et al. Etranacogene dezaparvovec (AMT-061 phase 2b): normal/near normal FIX activity and bleed cessation in hemophilia B Blood Advances. 2019;3(21):3241-47.
- Rodríguez-Merchán EC, et al. Gene Therapy in Hemophilia: Recent Advances. Int. J. Mol. Sci. 2021;22:7647-67.
- Miesbach W, et al. Gene therapy with adeno-associated virus vector 5–human factor IX in adults with hemophilia B. Blood. 2018;131(9):1022-31.
- Perrin GQ, Herzog RW, Markusic DM. Update on clinical gene therapy for Haemophilia. Blood. 2019;133(5):407-414.