
Are You a Healthcare Professional?
This European website, initiated and developed by CSL Behring, contains product specific information and promotional content on gene therapy in haemophilia B and is intended for an international audience of European healthcare professionals only. For more detailed information on the use of the product in your country, please visit www.cslbehring.com.
Yes, I am a healthcare professional*
Enter SiteClinical Evidence

Efficacy with a one-time infusion
HEMGENIX® helps people with haemophilia B realize a new level of freedom: a one-time infusion can provide long-term bleed protection and near normal, long-lasting Factor IX levels for a wide range of patients.1,3
Freedom from routine prophylaxis
96,3% of patients discontinued routine FIX prophylaxis and remained prophylaxis-free up to months 24 after administration of HEMGENIX®.1
52 of 54 patients remained free of previous continuous routine FIX prophylaxis through to month 24 post-treatment. 2 patients did not respond to HEMGENIX® treatment: 1 patient received only 10% of the planned dose and 1 patient had pre-existing AAV5 NAb titre of 1:3212.1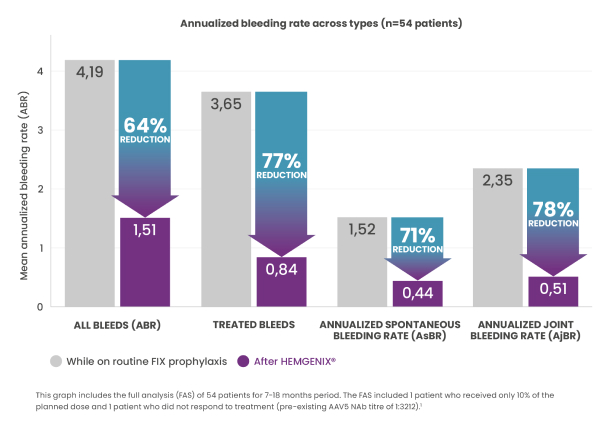
Long-term bleed protection
64% annualized bleeding rate (ABR) reduction vs well-conducted prophylaxis in lead-in period. All bleeding rates measured were significantly reduced following HEMGENIX® treatment.1,3
The ABR for all types of bleeds after stable FIX expression decreased from a mean of 4,19 for the lead-in period (all patients were under well-conducted prophylaxis) to a mean of 1,51 (p=0,0002) in the months 7–18 post-dose.1,3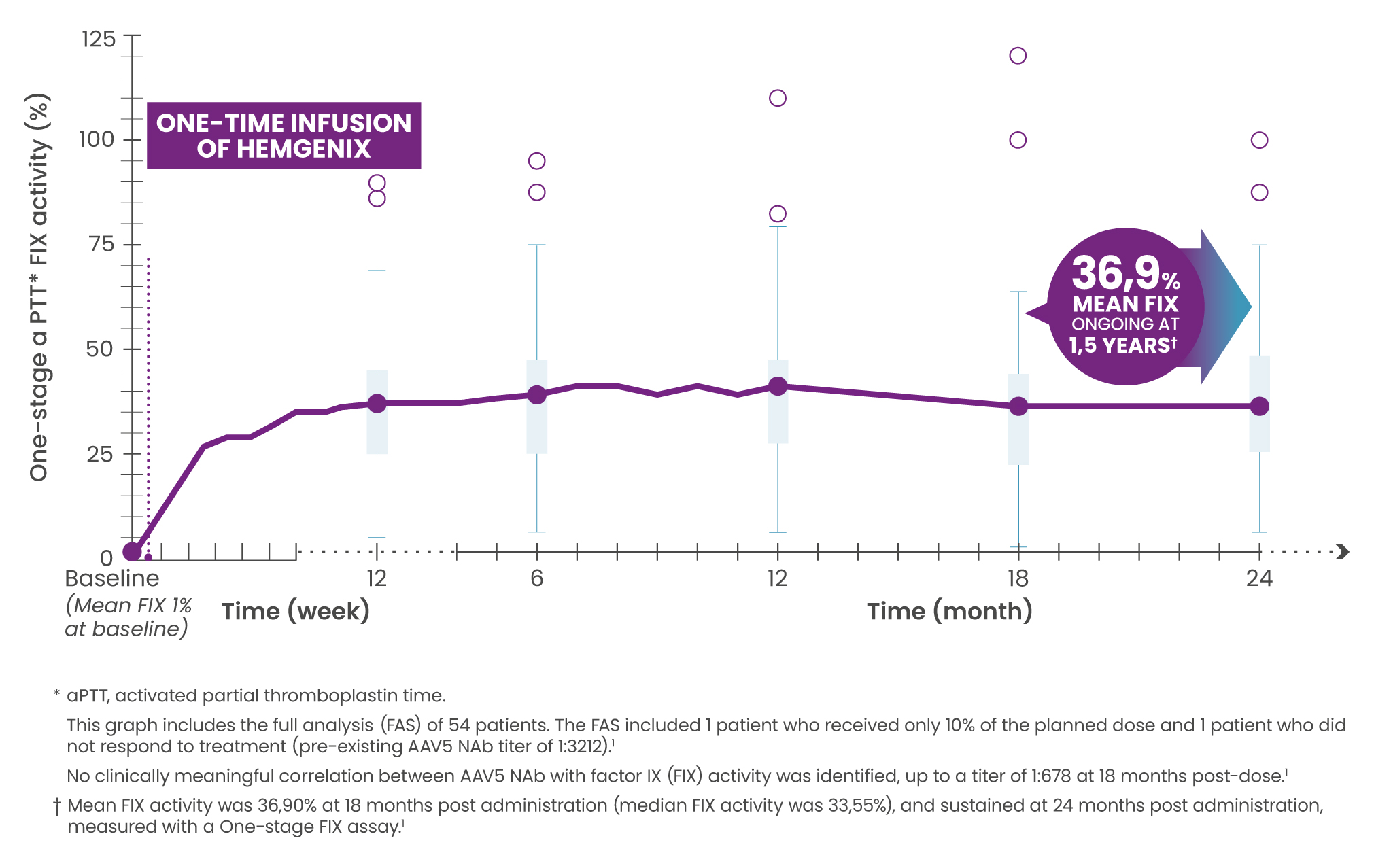
Elevated and sustained FIX activity
Elevated and sustained FIX activity
Mean FIX activity (n=50) was 36,90% (SD 21,40) at 18 months post administration (median FIX activity was 33,55%), and sustained at 24 months post administration, measured with One-stage FIX assay.1
High Success Rate
100% of patients (n=52) who responded to HEMGENIX® treatment discontinued routine FIX prophylaxis post-infusion and remained prophylaxis-free up to 24 months after HEMGENIX® administration.1
52 of 54 patients remained free of previous continuous routine FIX prophylaxis up to month 24 post treatment. 2 patients did not respond to HEMGENIX® treatment: 1 patient received only 10% of the planned dose and 1 patient had pre-existing AAV5 NAb titre of 1:3212.1
Life-changing Results
Patients experienced a statistically significant improvement in overall quality of life (p=<0,0001) and in the domains of feelings (p=<0,0001), treatment (p=<0,0001), work/school (p=0,0036) and future (p=0,0023).5
Safety Profile
HEMGENIX® was safe and effective in the pivotal phase 3 clinical trial, there were no treatment related serious adverse events and no development of FIX inhibitors reported.1,*
* For a full list of AEs, please see the Summary of Product Characteristics.
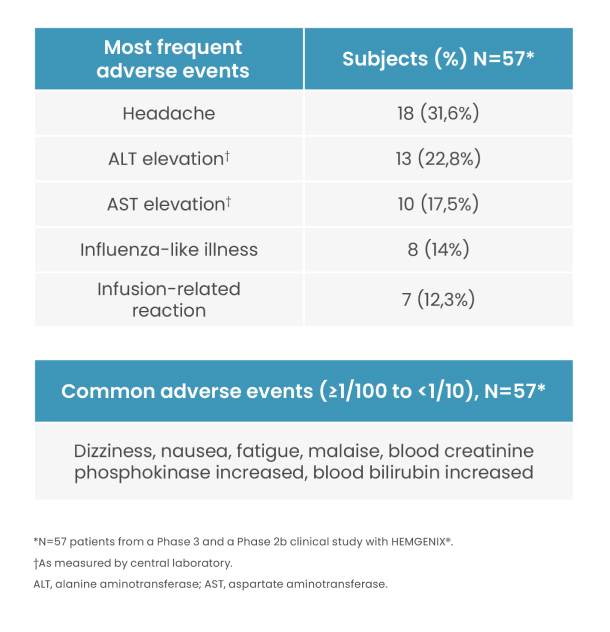
Adverse Events
Most frequent treatment-related adverse events were mild.3 Infusion-related reactions (IRR) occurred in 7/54 (12,3%) of patients, including hypersensitivity reactions and anaphylaxis, with the majority being mild and resolving on the same day.1
In the event of an IRR during administration, the infusion may be slowed or temporarily stopped and restarted at a slower rate once the IRR is resolved.1
Transient liver enzyme elevation
All treatment-related ALT elevations were non-serious and resolved with a course of corticosteroid treatment. Patients should be monitored weekly for liver enzyme elevations in the first three months following HEMGENIX® administration.1
In the clinical phase 2b and phase 3 (HOPE-B) studies, ALT-elevations occurred in 13/57 (22,8%) patients. Nine out of 13 patients with ALT elevations received a tapered course of corticosteroid. Mean duration of corticosteroid treatment for the elevated ALT was 81,4 days. All treatment-related ALT elevations were non-serious and resolved within 3 to 127 days. Prophylactic steroids to prevent ALT elevation were not required.1
FREQUENTLY ASKED QUESTIONS
Previous
About HEMGENIX®
Discover more about HEMGENIX® design, development and mechanism of action.
Next
Patient Experience
See the timeline of events experienced by haemophilia B patients on their road to HEMGENIX® treatment.
References
- EU SmPC HEMGENIX® (European Medicine Agency, 2023).
- CSL Behring. First Gene Therapy for Hemophilia B, CSL's HEMGENIX®, Approved by the European Commission. Press release. 20 Feb 2023. Available at: https://newsroom.csl.com/2023-02-20-First-Gene-Therapy-for-Hemophilia-B,-CSLs-HEMGENIX-R-,-Approved-by-the-European-Commission. Accessed March 2023.
- Pipe SW, et al. Gene Therapy with Etranacogene Dezaparvovec for Hemophilia B. N Engl J Med. 2023;388:706-718.
- von Drygalski A, Gomez E, Giermasz A, et al. Stable and durable factor IX levels in hemophilia B patients over 3 years post etranacogene dezaparvovec gene therapy. Blood Adv 2022; doi: https://doi.org/10.1182/bloodadvances.2022008886.
- Miesbach W, Leebeek FWG, Recht M, et al. Final analysis from the pivotal phase 3 HOPE-B gene therapy trial: stable steady-state efficacy and safety of etranacogene dezaparvovec in adults with severe or moderately severe haemophilia B. Presented at: European Association for Haemophilia and Allied Disorders; February 2022; Virtual.
- Kruzik A, et al. Prevalence of Anti-Adeno-Associated Virus Immune Responses in International Cohorts of Healthy Donors. Mol Ther Methods Clin Devel. 2019;14:126-33.
- Klamroth R, et al. Global Seroprevalence of Pre-existing Immunity Against AAV5 and Other AAV Serotypes in People with Hemophilia A. Hum Gene Ther. 2022;33(7-8):432-441.
- Falese L, et al. Strategy to detect pre-existing immunity to AAV gene therapy. Gene Ther. 2017;24(12):768-78.